The decomposition of N2O5(g) to NO2(g) and O2(g) obeys first-order kinetics. Assume the form of the rate law is
Rate 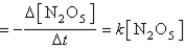 where k = 3.4 × 10-5 s-1 at 25°C.
where k = 3.4 × 10-5 s-1 at 25°C.
-What is the half-life for the reaction described?
Definitions:
CRM Program
Customer Relationship Management program; a strategy for managing an organization's interactions with current and potential customers using data analysis about customers' history with a company.
Customer's Profitability
The financial benefit a company gains from dealing with a customer, considering the revenues generated and the costs associated with maintaining the relationship.
Order Status Communication
The process of providing updates to customers about the progress and current status of their orders, from placement through to delivery.
Human Method
An approach that relies on human input, expertise, or interaction as part of a process or system.
Q8: A certain compound with a molar mass
Q13: [Cr(NH<sub>3</sub>)<sub>5</sub>Cl]SO<sub>4</sub><br>A) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB34225555/.jpg" alt="[Cr(NH<sub>3</sub>)<sub>5</sub>Cl]SO<sub>4</sub> A)
Q41: Predict the deviation from Raoult's law when
Q47: Which of the following is the oxidation
Q48: How many unpaired electrons are there in
Q53: Lithium metal with H<sub>2</sub>(g)
Q60: From the following list of observations, choose
Q71: A 5.22-g sample of a compound is
Q84: The standard potential for the reaction A(s)
Q109: Which of the following should have the