The reaction 2NO → N2 + O2 has the following rate law: 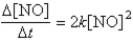 After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
After a period of 2.0 × 103 s, the concentration of NO falls from an initial value of 2.8 × 10-3 mol/L to 2.0 × 10-4 mol/L. What is the rate constant, k?
Definitions:
Residual Income
Income that continues to be generated after the initial effort has been expended, often used in the context of investments or intellectual property.
Minimum Required Rate of Return
The lowest acceptable return on an investment, determined by an investor's risk tolerance and other factors, used as a benchmark for evaluating potential investments.
Operating Assets
Cash, accounts receivable, inventory, plant and equipment, and all other assets held for operating purposes.
Margin
The difference between the selling price of a product or service and its production or acquisition cost, often expressed as a percentage.
Q2: What is the hybridization of the carbon
Q7: For which element are the d orbitals
Q19: Complete the Lewis structure for the following
Q47: Na
Q69: The following question refers to the gas-phase
Q78: If an electrolysis plant operates its electrolytic
Q78: What is the hybridization of the central
Q81: An excess of finely divided iron is
Q84: The standard potential for the reaction A(s)
Q92: Consider the reaction<br>3A + B + C