For the reaction
CH3CHCH2(g) + HCl(g) → CH3CHClCH3(g)
a possible mechanism is 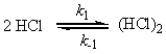
Fast equilibrium 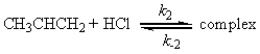
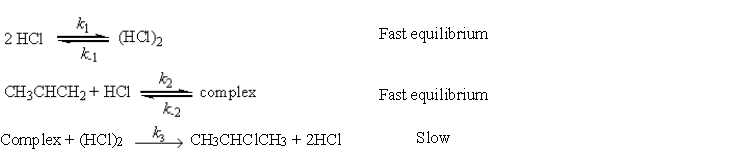
Fast equilibrium
Complex + (HCl)2 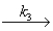
CH3CHClCH3 + 2HCl
Slow
Derive the rate law for this reaction using this mechanism.
Definitions:
General Adaptation Syndrome
A theory by Hans Selye that describes the three stages of response to stress: alarm, resistance, and exhaustion.
Catharsis
The process of releasing and thereby providing relief from strong or repressed emotions, often through art, dialogue, or reflection.
Self-Esteem
A person's personal and subjective assessment of their own value in terms of emotions.
Individualism
A principle favoring freedom of action for individuals over collective or state control.
Q2: Identify the true statement(s) of potassium's electronic
Q20: The reaction A → B + C
Q22: The OH radical disproportionates according to the
Q32: Specify the hybridization of the nitrogen atom
Q42: You have a 10.40-g mixture of table
Q44: Choose the element with the highest melting
Q45: The reduction potentials for Au<sup>3+</sup> and Ni<sup>2+</sup>
Q63: Calculate the mole fraction of KCl in
Q70: How many oxides of carbon are there?<br>A)
Q92: The lattice energy of NaI is -686