Given the following information: 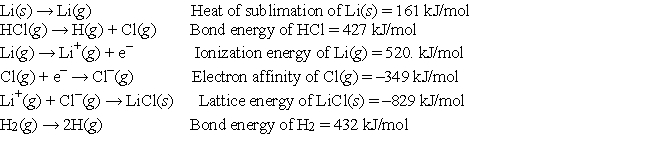 calculate the net change in energy for the reaction 2Li(s) + 2HCl(g) → 2LiCl(s) + H2(g)
calculate the net change in energy for the reaction 2Li(s) + 2HCl(g) → 2LiCl(s) + H2(g)
Definitions:
Symmetry-Allowed
In quantum chemistry, a process or transition is symmetry-allowed if it does not violate symmetry rules governing molecular transformations.
Reaction Outcome
The final products, yields, and implications of a chemical reaction based on the reactants and conditions employed.
Major Organic Product
The primary substance produced with the highest yield in a reaction involving organic compounds.
Organic Reaction
A chemical reaction involving organic compounds, leading to the transformation or creation of new organic molecules.
Q3: Which of the following statements is correct?<br>A)
Q21: For how many of the following does
Q30: hexane (C<sub>6</sub>H<sub>14</sub>) and chloroform (CHCl<sub>3</sub>)<br>A) relatively ideal<br>B)
Q56: Which molecule or ion violates the octet
Q56: Which of the following electron distributions among
Q67: Calculate [A] at t = 81 min.<br>A)
Q69: Calculate the osmotic pressure (in torr) of
Q85: For the reaction 2N<sub>2</sub>O<sub>5</sub>(g) → 4NO<sub>2</sub>(g) +
Q87: What form will the pseudo-rate law have?<br>A)
Q123: What is the concentration of C after