The following questions refer to a galvanic cell that utilizes the following reaction (unbalanced) :(AuCl4) -(aq) + Cu(s) → Au(s) + Cl-(aq) + Cu2+(aq)
-Given the following information, determine the standard cell potential. 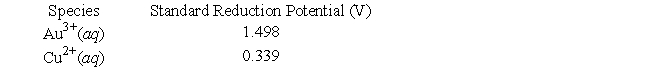
Definitions:
Lockdown Mode
A security feature on devices that restricts the functionality and access to data to protect against potential threats.
Ethical Outcomes
Results or consequences of actions that are considered morally right and beneficial in terms of ethical standards.
Legal Outcomes
The results or consequences of legal actions or proceedings.
Apologize
The act of expressing regret or sorrow for having done something wrong or caused inconvenience to others.
Q3: Choose the electron dot formula that most
Q18: What is the value of the rate
Q27: Calculate ΔS.<br>A) -20.5 J/K<br>B) -26.8 J/K<br>C) 26.8
Q30: Assuming ΔH° and ΔS° are temperature independent,
Q33: Consider the reaction<br>3A + B + C
Q40: When 0.157 mol of NH<sub>3</sub> is reacted
Q54: A calorimeter contains 240 g of water
Q82: Refer to the galvanic cell below (the
Q98: What is ΔG° at 25°C?<br>A) +2300 J<br>B)
Q177: Calculate the concentration of chromate ion, CrO<sub>4</sub><sup>2-</sup>,