Choose the correct statement(s) given the following information: 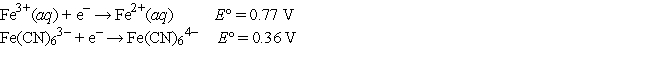
I.Fe2+(aq) is more likely to be oxidized than Fe2+ complexed to CN-.
II.Fe3+(aq) is more likely to be reduced than Fe3+ complexed to CN-.
III.Complexation of Fe ions with CN- has no effect on their tendencies to become
Oxidized or reduced.
Definitions:
Q14: Two metals of equal mass with different
Q15: An electrolytic cell process involves plating Zr(s)
Q52: For which of the following can we
Q64: What is true about the value of
Q85: For the reaction 2N<sub>2</sub>O<sub>5</sub>(g) → 4NO<sub>2</sub>(g) +
Q106: What is the order of the reaction
Q112: For which of the following processes would
Q117: Which of the following statements is/are true
Q122: Consider the reaction below at 25°C, for
Q171: Which of the following statements is/are true