Refer to the galvanic cell below (the contents of each half-cell are written beneath each compartment) . 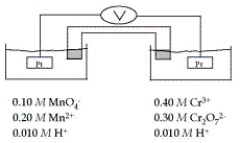 The standard reduction potentials are as follows:
The standard reduction potentials are as follows: 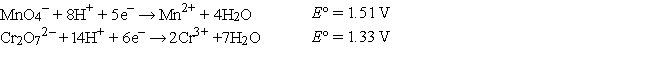
-In the balanced cell reaction, what is the stoichiometric coefficient for H+?
Definitions:
Traditional Negotiating
A bargaining approach typically involving two or more parties seeking to reach an agreement on terms or conditions through discussion and compromise.
Kaizen
A Japanese philosophy that focuses on continuous improvement in all aspects of life, including business and manufacturing processes.
Nadler-Tushman Model
A framework for diagnosing organizational behavior and performance, focusing on the interactions among strategy, structure, and organizational processes.
Organizational Change
The process through which companies or institutions modify existing processes, culture, or structures to achieve new objectives or improve performance.
Q6: What is the hybridization of each N
Q26: For a certain process, at 319 K,
Q45: Calculate ΔS for cooling 2.60 mol of
Q50: Select the correct molecular structure for IF<sub>6</sub><sup>+</sup>.<br>A)
Q58: Chromate ion is added to a saturated
Q73: How many seconds would it take to
Q73: Select the correct molecular structure for PO<sub>4</sub><sup>3-</sup>.<br>A)
Q75: What is the electron configuration for the
Q85: CH<sub>4</sub> + 4Cl<sub>2</sub>(g) → CCl<sub>4</sub>(g) + 4HCl(g),
Q92: For this system at equilibrium, how will