Calculate E at 25°C for this cell, given the following data: 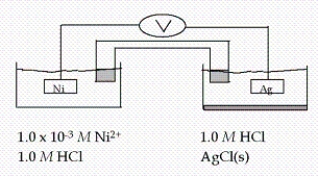 Ag+ + e- → Ag(s) E° = 0.80 V
Ag+ + e- → Ag(s) E° = 0.80 V
Ni2+ + 2e- → Ni(s) E° = -0.23 V
Ksp for AgCl = 1.6 × 10-10
Definitions:
Trade Credit
A type of commercial financing in which a customer is allowed to purchase goods or services and pay the supplier at a later scheduled date.
Commercial Paper
A short-term, unsecured debt security that corporations issue, primarily to fund payroll, accounts payable, and inventory needs.
Bank Loans
Debt provided by banking institutions that is repayable over a set period of time with interest.
Pledged
Assets that are promised or committed as security for the fulfillment of a debt or an obligation.
Q2: Identify the true statement(s) of potassium's electronic
Q3: The combustion of methanol takes place according
Q10: The equilibrium constant for the reaction, Is
Q52: For which of the following can we
Q65: For the reaction<br>2NO(g) + H<sub>2</sub>(g) → N<sub>2</sub>O(g)
Q72: Which reaction does not proceed far to
Q101: Calculate ΔG° for the reaction at 25°C.<br>A)
Q105: Identify the order that is observed experimentally
Q114: How many moles of HCl(g) must be
Q155: Which titration curve would result from the