Which of the following result(s) in an increase in the entropy of the system? 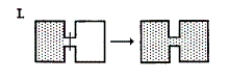 I. (See diagram.)
I. (See diagram.)
II. Br2(g) → Br2(l)
III. NaBr(s) → Na+(aq) + Br-(aq)
IV. O2(298 K) → O2(373 K)
V. NH3(1 atm, 298 K) → NH3(3 atm, 298 K)
Definitions:
Variable Cost
Costs that change in proportion to the goods or services that a business produces.
General Transfer-Pricing Rule
A framework or methodology used to determine the price for goods or services transferred between divisions or subsidiaries of the same company.
Goal Congruence
A condition in which the goals of the organization's different members or departments align and support the overall objectives of the organization.
Perfect Competition
An ideal market scenario in which there are many buyers and sellers, homogeneous products, and no barriers to entry or exit.
Q7: For which element are the d orbitals
Q8: Consider the following reaction:<br>2Al(s) + 3Cl<sub>2</sub>(g) →
Q14: Which of the following statements is false?<br>A)
Q30: What is true about the value of
Q38: Which species has an unpaired electron?<br>A) OH<sup>-</sup><br>B)
Q45: The reduction potentials for Au<sup>3+</sup> and Ni<sup>2+</sup>
Q57: An electron in a 10.0-nm one-dimensional box
Q61: Calculate ΔV.<br>A) -5.87 L<br>B) 6.11 L<br>C) -6.11
Q77: According to the VSEPR model, the electron
Q100: Given the following free energies of formation: