Which of the following result(s) in an increase in the entropy of the system? 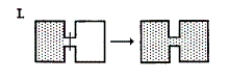 I. (See diagram.)
I. (See diagram.)
II. Br2(g) → Br2(l)
III. NaBr(s) → Na+(aq) + Br-(aq)
IV. O2(298 K) → O2(373 K)
V. NH3(1 atm, 298 K) → NH3(3 atm, 298 K)
Definitions:
Saturated Fat
A type of dietary fat found primarily in animal products, which is solid at room temperature and can raise cholesterol levels.
Behavioral Smoking Cessation Programs
Programs designed to help individuals stop smoking by modifying their behavior and coping strategies.
Lung Cancer
A type of cancer that begins in the lungs, often associated with smoking and tobacco use.
Heart Disease
A range of conditions that affect the heart, such as coronary artery disease, arrhythmias, and congenital heart defects.
Q18: Cu<sup>2+</sup> + 2e<sup>-</sup> → Cu(s) E° =
Q27: An element with the electron configuration [Xe]4f<sup>14</sup>5d<sup>7</sup>6s<sup>2</sup>
Q48: The activation energy for the reaction H<sub>2</sub>(g)
Q53: If an acid, HA, is 10.0% dissociated
Q63: Calculate [H<sup>+</sup>] after 300.0 mL of 1.00
Q95: In 6 M HCl, the complex ion
Q106: ΔS<sub>surr</sub> is _ for exothermic reactions and
Q119: What type of structure does the XeOF<sub>2</sub>
Q130: After 0 mL of 0.200 M NaOH
Q173: Consider a solution of 2.0 M HCN