Multiple Choice
Consider the following hypothetical reaction (at 307 K) . Standard free energies, in kJ/mol, are given in parentheses. 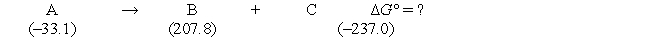 What is the value of the equilibrium constant for the reaction at 307 K?
What is the value of the equilibrium constant for the reaction at 307 K?
Definitions:
Related Questions
Q2: Calculate the pH of a solution made
Q5: From the following list of observations, choose
Q9: After 75.0 mL of 0.200 M NaOH
Q15: A 1.00-g sample of the rocket fuel
Q21: A gas releases 2.0 J of heat
Q41: A calorimeter contains 142 g of water
Q51: The standard free energy of formation of
Q59: The standard molar free energies of formation
Q96: In an isothermal process, the pressure on
Q117: Which of the following statements is/are true