A gas phase decomposition reaction
2AB3 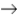 A2 + 3B2
A2 + 3B2
Is exothermic. What can you conclude about the spontaneity of this reaction?
Definitions:
Equilibrium Constant
A numerical value that represents the ratio of concentrations of products to reactants at equilibrium, for a reversible chemical reaction.
Equilibrium
A state in which all acting influences are balanced or canceled by equal opposing forces, resulting in a stable system.
Reactants
Ingredients that are present from the start in a chemical reaction, getting used up as they generate the outcome products.
Equilibrium
The state in which both reactants and products are present in concentrations which have no further tendency to change with time, usually in a closed system.
Q3: Once equilibrium was established, some additional chlorine
Q6: At a given temperature, the equilibrium constant
Q10: A 50.0-mL solution of the acid H<sub>3</sub>A
Q14: Which of the following statements is false?<br>A)
Q16: When current is allowed to flow, which
Q39: Which of the following shows these molecules
Q42: Of the following elements, which has occupied
Q54: Which of the following molecules has a
Q60: Which of the following molecules contains a
Q96: How many Lewis structures does CO<sub>3</sub><sup>2-</sup> have?<br>A)