Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 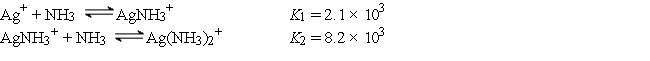 The concentration of Ag(NH3) 2+ at equilibrium is
The concentration of Ag(NH3) 2+ at equilibrium is
Definitions:
Dystrophin
A protein involved in maintaining the integrity of muscle cells, with mutations in its gene leading to muscular dystrophies.
Muscular Dystrophy
A group of genetic diseases characterized by progressive weakness and degeneration of the skeletal muscles.
Protein Lack
A nutritional deficiency state where there is an inadequate intake of proteins, leading to health issues such as muscle wasting and immune system dysfunction.
Duplication
Change in chromosome structure in which a particular segment is present more than once in the same chromosome.
Q1: A 0.210-g sample of an acid (molar
Q9: Using the following data, calculate the standard
Q26: Which of the following statements is/are true
Q36: In the _ process, the heavier molecules
Q42: Calculate the pH of a 2.0 ×
Q48: 1.0 M NaHSO<sub>4</sub> (pK<sub>a</sub> for HSO<sub>4</sub><sup>-</sup> =
Q54: Roundup, an herbicide manufactured by Monsanto, has
Q68: Identify the strongest acid.<br>A) HNO<sub>3</sub><br>B) HCN<br>C) OH<sup>-</sup><br>D)
Q87: The sodium salt, NaA, of a weak
Q88: What is the value of Q, the