Multiple Choice
Given the following values of equilibrium constants: 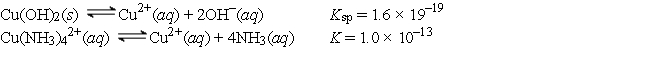 What is the value of the equilibrium constant for the following reaction?
What is the value of the equilibrium constant for the following reaction?
Cu(OH) 2(s) + 4NH3(aq) 
Cu(NH3) 42+(aq) + 2OH-(aq)
Definitions:
Related Questions
Q11: In which direction do electrons flow in
Q22: Consider an electrochemical cell with a copper
Q24: If, at a given temperature, the equilibrium
Q26: The ratio of the forces per wall
Q36: In the _ process, the heavier molecules
Q38: Given the reaction A(g) + B(g) <img
Q46: Calculate the pH of the final solution
Q55: Which statement is true of a process
Q57: Determine the standard potential, E°, of a
Q104: A 130.-mL sample of gas is collected