Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 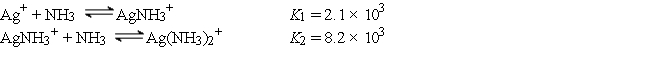 The concentration of Ag+ at equilibrium is
The concentration of Ag+ at equilibrium is
Definitions:
Adult Criminals
Individuals over the age of legal adulthood who engage in activities considered to be illegal or criminal by law.
Aggressive Interactions
Aggressive interactions are exchanges between individuals characterized by hostility, conflict, or intentions to harm one another.
Only-born Adults
Adults who were an only child during their upbringing, potentially affecting their personality and social interactions.
Self-esteem
The subjective evaluation or appraisal of one's own worth, encompassing beliefs about oneself as well as emotional states.
Q12: Consider three 1-L flasks at the same
Q22: ΔS<sub>surr</sub> for this process is<br>A) less than
Q32: A glass column is filled with mercury
Q48: 1.0 M NaHSO<sub>4</sub> (pK<sub>a</sub> for HSO<sub>4</sub><sup>-</sup> =
Q65: Balance the reaction below in acidic solution.IO<sub>3</sub><sup>-</sup>
Q66: Determine the number of electrons transferred during
Q70: A 50.0-g sample of a metal is
Q70: What is ΔS?<br>A) More information is needed.<br>B)
Q75: Consider the equation 2A(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider
Q93: Consider the titration of 200.0 mL of