The Ag+ ion reacts with NH3 to form the following complex ions: 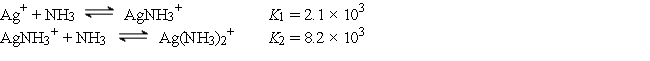
AgCl (Ksp = 1.6 × 10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
Definitions:
Economic Recession
A period of temporary economic decline during which trade and industrial activity are reduced, generally identified by a fall in GDP in two successive quarters.
Homogeneous Products
Products that are essentially identical in nature and can easily be substituted for one another, typically found in highly competitive markets.
Undifferentiated Selling
describes a marketing strategy where a company offers the same product or message to all potential customers, without segmentation or personalization.
Homogeneous Selling
A sales approach where the products or services offered are uniform or very similar in characteristics.
Q1: Which of the following determines the equilibrium
Q23: Find the pH of a solution at
Q27: It is found that 250. mL of
Q40: An excess of potassium hydroxide is treated
Q59: Gold (atomic mass = 197 g/mol) is
Q61: Calculate [H<sup>+</sup>] in a 1.2 × 10<sup>-5</sup>
Q63: A 0.050 M aqueous solution of a
Q65: If you know K<sub>b</sub> for ammonia, NH<sub>3</sub>,
Q77: Calculate the ratio of impacts with the
Q129: Water gas, a commercial fuel, is made