A 50.00-mL sample of 0.100 M Ca(NO3) 2 is mixed with 50.00 mL of 0.200 M NaF. When the system has come to equilibrium, which of the following sets of conditions will hold? The Ksp for CaF2 is 4.0 × 10-11. 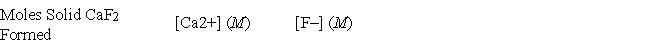
Definitions:
Forward Buy
A purchasing strategy where a buyer purchases larger quantities of goods in advance to take advantage of lower prices or to hedge against future price increases.
Profitability
The ability of a business to generate more revenue than the expenses incurred, resulting in a profit.
Manufacturing Capacity
The maximum amount of products a factory or production facility can produce within a given time period.
Inventory Costs
Expenses associated with holding and managing goods or materials until they are sold or used in production.
Q24: If, at a given temperature, the equilibrium
Q31: The value of K<sub>p</sub> for the reaction
Q45: Determine the pH of a 7.5 M
Q53: A 100.0-mL sample of 0.2 M (CH<sub>3</sub>)<sub>3</sub>N
Q68: Identify the strongest acid.<br>A) HNO<sub>3</sub><br>B) HCN<br>C) OH<sup>-</sup><br>D)
Q94: For the reaction of sodium bromide with
Q95: The solubility of AgCl in water is
Q102: Consider the ideal pressure equation for gases,
Q130: After 0 mL of 0.200 M NaOH
Q140: Given that ΔG°<sub>f</sub> for NH<sub>3</sub> = -16.67