Consider a solution made by mixing 500.0 mL of 4.0 M NH3 and 500.0 mL of 0.40 M AgNO3. Ag+ reacts with NH3 to form AgNH3+ and Ag(NH3) 2+: 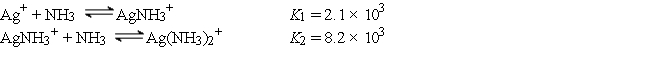 The concentration of Ag+ at equilibrium is
The concentration of Ag+ at equilibrium is
Definitions:
Specific Environment
The part of the environment that is directly relevant to an organization, including customers, suppliers, competitors, and government regulations.
Government Agencies
Organizations established by a government to administer legislation and implement public policy in specific areas.
Legal-Political Conditions
The framework of laws and political climate within which individuals, organizations, and businesses operate.
General Environment
The broad external conditions that affect organizations and their operations, including economic, legal, political, social, and technological factors.
Q5: The reaction<br>H<sub>2</sub>(g) + I<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="The
Q19: The following reaction occurs in aqueous acid
Q31: What volume of 0.436 M barium nitrate
Q32: Calculate the work for the expansion of
Q47: Consider the reaction<br>CaCl<sub>2</sub>(s) + 2H<sub>2</sub>O(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg"
Q51: Which of the following would be a
Q60: In a common car battery, six identical
Q75: Consider the equation 2A(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider
Q87: What is the molar mass of ethanol
Q96: Which statements about hydrogen are true?<br>I. H