The Ag+ ion reacts with NH3 to form the following complex ions: 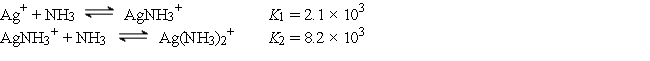
AgCl (Ksp = 1.6 × 10-10) is dissolved to its solubility limit in 10.0 M NH3. Calculate the equilibrium concentrations of Ag+, Cl-, Ag(NH3)2+, and NH3.
Definitions:
Health Care Professionals
Individuals who provide medical services and care to patients, including doctors, nurses, therapists, and pharmacists.
SMART Approach
An acronym that stands for Specific, Measurable, Achievable, Relevant, and Time-bound, commonly used in goal setting to ensure objectives are clear and attainable.
Outcome Statements
Concise descriptions of the specific results that are expected to be achieved from a healthcare intervention or treatment.
Patient Outcome
The results or end points of medical care, which measure the effectiveness of treatments or interventions on a patient's health status.
Q1: Calculate the pH of the following aqueous
Q11: At a certain temperature, K for the
Q14: Consider two samples of helium in separate
Q19: Calculate the equilibrium concentration of N<sub>2</sub>.<br>A) 1.5
Q28: A sample contains 12.0 moles of a
Q45: Determine the pH of a 7.5 M
Q45: What is the pH of this solution?<br>A)
Q61: The heat combustion of acetylene, C<sub>2</sub>H<sub>2</sub>(g), at
Q74: After 300.0 mL of 0.200 M NaOH
Q76: Assume that the enthalpy of fusion of