Consider the following reaction: 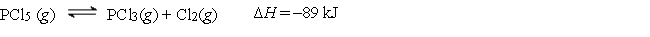
-How can the equilibrium be shifted to the right?
Definitions:
Metabolic Acidosis
A medical condition characterized by an increase in acidity in the blood and body tissues, often resulting from kidney disease, uncontrolled diabetes, or excessive alcohol intake.
pH Level
A measure of acidity or alkalinity of a solution, with 7 being neutral, values below 7 acidic, and values above 7 alkaline.
Roy's Model
A nursing theory that focuses on the adaptation of the individual to changes in environment and health status.
Spirituality
Belief in or relationship with some higher power, creative force, driving being, or infinite source of energy.
Q29: At room temperature cyclohexane exists almost exclusively
Q35: The standard enthalpy change for the following
Q48: 1.0 M NaHSO<sub>4</sub> (pK<sub>a</sub> for HSO<sub>4</sub><sup>-</sup> =
Q50: The DETERMINE acronym refers to all of
Q55: What concentration of HF (K<sub>a</sub> = 7.2
Q62: Calculate the pH of a 2.0 ×
Q77: The ΔH value for the reaction (1/2)O<sub>2</sub>(g)
Q87: [Cu(CN)<sub>3</sub><sup>2-</sup>]<br>A) 3.33 M<br>B) 1.67 M<br>C) 2.0 ×
Q95: Consider the reaction<br>2NO<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6422/.jpg" alt="Consider the
Q119: The cation M<sup>2+</sup> reacts with NH<sub>3</sub> to