Based on the van der Waals equation of state 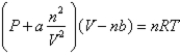 Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas.
Which of the following molecules - hydrogen chloride, nitrogen dioxide, or sulfur dioxide - should behave most like an ideal gas. 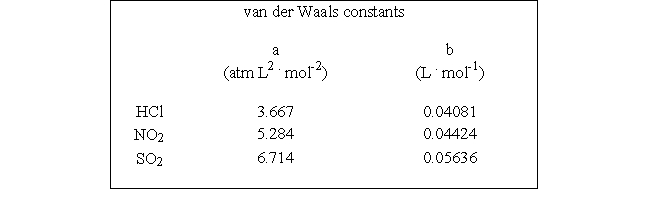
Definitions:
Consumer Price Index
A measure that examines the weighted average of prices of a basket of consumer goods and services, often used as an indicator of inflation.
Pretzels
A type of baked bread product made from dough most commonly shaped into a twisted knot.
Cookies
Small pieces of data sent from a website and stored on a user's computer by the user's web browser while the user is browsing, often used to remember information about the user.
Information Asymmetry
A situation where one party in a transaction has more or better information than the other, often leading to an imbalance in power or unfair advantage.
Q14: What is the net ionic equation for
Q26: The following unbalanced reaction occurs in basic
Q38: The two salts AgX and AgY have
Q40: You are titrating a solution of sodium
Q44: Identify the potential fuel that can be
Q46: What is the pH of a 0.2
Q91: Balance the reaction below in acidic aqueous
Q96: Which of the following solutions contains the
Q121: Calculate the pH of a 1.8 M
Q126: A 75.0-mL sample of 0.0500 M HCN