The potential energy for the interaction between the two atoms in a diatomic molecule is U = A/x12 - B/x6, where A and B are constants and x is the interatomic distance. The magnitude of the force that one atom exerts on the other is: 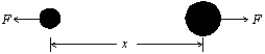
Definitions:
Cystic Fibrosis
A genetic disorder affecting the lungs and other organs, characterized by thick, sticky mucus that can block airways.
Carbon Monoxide Poisoning
A potentially fatal condition caused by inhaling carbon monoxide gas, leading to symptoms such as headache, dizziness, and nausea, due to reduced oxygen carrying capacity of blood.
Smoke Inhalation
The act of breathing in combustion products during exposure to fire, which can lead to respiratory distress and other health issues.
Q2: Suppose that the fundamental dimensions are taken
Q17: A block of mass m is
Q22: The two arms of a U-tube are
Q35: A stone is released from rest from
Q60: In Korea, how did religious practice change
Q64: A freely falling body has a constant
Q65: Consider the following five graphs (note the
Q70: At time t = 0 a car
Q74: A thin rod of length L has
Q77: An object hangs from a spring balance.