The potential energy for the interaction between the two atoms in a diatomic molecule is U = A/x12 - B/x6, where A and B are constants and x is the interatomic distance. The magnitude of the force that one atom exerts on the other is: 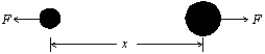
Definitions:
Canadian Life Expectancy
The average number of years that individuals born in Canada are expected to live, based on statistical averages.
Health Programs
Initiatives sponsored by governments, private institutions, or communities aimed at improving health and preventing disease.
Lifespan
The average period that a living being or organism is expected to live.
Pathologization
The process of treating or characterizing behaviors, beliefs, or conditions as indicative of a disease or disorder.
Q5: The vectors , , and are related
Q6: A projectile is fired straight upward from
Q9: A stationary mass m = 1.3 kg
Q13: Two bodies are falling with negligible air
Q35: A wheel rotates with a constant
Q42: One end of a cylindrical pipe has
Q56: When a 40-N force, parallel to
Q57: A fluid is undergoing "incompressible" flow. This
Q62: A particle, held by a string whose
Q62: A particle starts from rest and is