Consider the Following Image Representing an Atom Particles by This Nucleus?
A)The Number of Protons Would Increase
Consider the following image representing an atom. 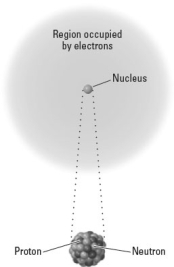 Which of the following would describe the net effect of the emission of a particles by this nucleus?
Which of the following would describe the net effect of the emission of a particles by this nucleus?
Definitions:
Model Business Corporation Act
A model set of laws prepared by legal experts to guide states in the development of their corporate statutes.
Shares
Units of ownership in a corporation or financial asset that represent an equal proportion of its capital.
Benefit
An advantage, profit, or gain received or enjoyed by an individual or entity, often as a result of a contract, employment, or other agreement.
Promoter
An individual or company who takes initiative to organize, finance, or promote and start up a new business venture or event.
Q4: A canister holds a gas at 16.3
Q5: If the position of equilibrium shifts to
Q8: The graph below represents a temperature versus
Q10: Examine the model of a form of
Q14: Consider the following general wedge-and-dash diagrams. (i)<img
Q15: Which of the following statements is false?<br>A)Gas
Q22: Which of the following are elements?<br>(i)KOH<br>(ii)Ne<br>(iii)CO<br>(iv)Carbon tetrachloride<br>(v)Calcium<br>A)i,ii,and
Q34: Considering the molecular mass and polarity influences
Q42: Write the equation for the complete oxidation
Q44: The maximum number of electrons that can