Identify the oxidation half-reaction in the redox equation:
Cu(s) + Cl2(g) 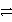
Cu2+ + 2 Cl-
Definitions:
Lifestyles
A way of living or doing things specific to an individual or group, often indicating consumption patterns and choices.
Marketers
Professionals who are involved in the promotion and selling of products or services, including market research and advertising strategies.
Flight Attendant
An airline employee responsible for ensuring the safety, security, and comfort of passengers aboard flights.
Commercial Aircraft
A type of aircraft designed and used for transporting passengers and cargo for business purposes.
Q1: Which of the following nonbankruptcy alternatives is
Q2: Given the following electronegativities: H = 2.1,F
Q4: Which of the following statements about atomic
Q5: The structures of cyclohexane and benzene are
Q12: Which of the following is true of
Q24: Aluminum hydroxide is added to nitric acid.Write
Q29: Which of the following are empirical formulas?<br>(i)C<sub>10</sub>H<sub>8</sub><br>(ii)CH<sub>2</sub><br>(iii)Al<sub>2</sub>Cl<sub>6</sub><br>(iv)NO<sub>2</sub><br>(v)Hg<sub>2</sub>Cl<sub>2</sub><br>A)i
Q31: Consider the following general structure. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg"
Q37: Which of the following bases is not
Q50: Which of the following statements is/are correct?<br>(i)Principal