Which of the following is the correct net ionic equation for the redox reaction between nickel ion and metallic silver and the correct direction that is favored? The half reactions are:
Ag+ + e- 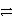
Ag(s) Ni2+ + 2e- 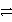
Ni(s) Ag+ is a stronger oxidizing agent than Ni2+.
Definitions:
Relative Change
The measure of change in a variable relative to its initial value, often expressed as a percentage.
Inelastic
Describes a situation where the quantity demanded or supplied of a good or service changes by a relatively small amount in response to changes in its price.
Quantity Demanded
The amount of a product or service consumers are willing and able to buy at a given price over a specified period of time.
Unit Elasticity
Demand or supply for which the elasticity coefficient is equal to 1; means that the percentage change in the quantity demanded or quantity supplied is equal to the percentage change in price.
Q1: When 2.77 g of a solute was
Q4: The energy of a photon of light
Q13: Cu(OH)<sub>2</sub> can be obtained from the addition
Q19: Examine the following two beakers. <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg"
Q20: Which of the following chapters allows a
Q22: Which of the following compounds is/are (an)ether(s)?<br>(i)CH<sub>3</sub>-CO-CH<sub>3</sub><br>(ii)CH<sub>3</sub>CH<sub>2</sub>-OH<br>(iii)C<sub>2</sub>H<sub>5</sub>-O-C<sub>2</sub>H<sub>5</sub><br>(iv)CH<sub>3</sub>(CH<sub>2</sub>)<sub>5</sub>-OH<br>(v)CH<sub>3</sub>-CH<sub>3</sub><br>A)i
Q25: A tenant's fixture can be removed by
Q29: For tax purposes, fixtures are treated as
Q36: Balance the following redox equation in acidic
Q42: A Brønsted-Lowry acid is defined as a(n)...<br>A)proton