The structural formula for glycerine is shown below.Compare the intermolecular forces in glycerine with those in n-hexane,C6H14,in which all carbons are in a continuous chain.Which of the following statements is true? 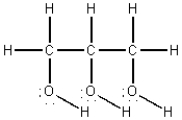
Definitions:
College Exam
A formal assessment or examination conducted in a college setting to evaluate a student's knowledge, skills, or academic achievement in a specific course or subject.
Improve Study Skills
Strategies and practices aimed at enhancing the effectiveness and efficiency of learning, including time management, note-taking, and active reading techniques.
Professor
An academic title for a senior teacher or lecturer, typically in a college or university.
Positive Terms
Typically words or phrases that convey affirmative, optimistic, or positive connotations or meanings.
Q3: In 2005, Congress adopted the Bankruptcy Reform
Q5: Predict the order in which boiling points
Q9: What is the oxidation number of titanium
Q11: A surety bond that ensures a property
Q13: How many liters of hydrogen,measured at
Q15: A mortgage is a security in which
Q18: The general rule of accession is that
Q22: Which of the following statements is incorrect?<br>(i)The
Q39: Choose the correct nuclear symbol for
Q46: Consider the particulate-level illustration: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6511/.jpg" alt="Consider