Consider the following thermochemical equation: CaO(s) + H2O( 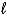 ) Ca(OH) 2(s) H = -66.5 kJ Which of the following statements is/are correct?
) Ca(OH) 2(s) H = -66.5 kJ Which of the following statements is/are correct?
(i) The reaction is exothermic
(ii) The reaction releases 66.5 kJ per gram of Ca(OH) 2(s) formed
(iii) Alternatively,the H term could be added to the product side of the equation
Definitions:
The process of producing a physical copy of documents, images, or other outputs from a computer or other digital device to paper or other media.
Presentation Theme
A visual scheme of colors, fonts, and effects applied to a presentation to maintain consistency and visual appeal.
Underline Button
A feature commonly found in text editing software that allows users to underline selected text for emphasis or stylistic purposes.
Format
The arrangement, structure, or organization of data, documents, or media according to specific guidelines or standards.
Q4: Consider the following general wedge-and-dash diagram: <img
Q5: In case of limited liability limited partnerships
Q6: The English Parliament enacted the Bubble Act
Q7: Consider the following beakers.In the beaker on
Q11: The Securities and Exchange Commission is bipartisan:
Q21: Under statutes enacted in many states, a
Q22: The Railway Labor Act established the _
Q23: Under Revised Uniform Partnership Act RUPA), dissolution
Q31: Which of the following has a trigonal
Q36: When coasting on a bicycle on a