Consider the following thermochemical equation: CaO(s) + H2O( 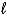 ) Ca(OH) 2(s) H = -66.5 kJ Which of the following statements is/are correct?
) Ca(OH) 2(s) H = -66.5 kJ Which of the following statements is/are correct?
(i) The reaction is exothermic
(ii) The reaction releases 66.5 kJ per gram of Ca(OH) 2(s) formed
(iii) Alternatively,the H term could be added to the product side of the equation
Definitions:
Technical Terms
Specialized words or phrases used in a specific field that may not be understood outside of that context.
Ramifications
The consequences or outcomes that result from a particular action or event, often complex or unexpected.
Technical Errors
Mistakes or problems that occur in the process of executing a technical task or operation, often related to machinery, computers, or specific techniques.
Psychological Obstacles
Internal barriers or mental hindrances that impede a person's ability to achieve goals or make beneficial decisions.
Q11: What is the conjugate base of ammonia?<br>A)NH<sub>3</sub>(g)<br>B)NH<sub>4</sub><sup>+</sup>(aq)<br>C)NH<sub>4</sub>OH(aq)<br>D)NH<sub>2</sub><sup>-</sup>(aq)<br>E)OH<sup>-</sup>(aq)
Q11: Under the zone of risk test an
Q14: Which of the following correctly matches each
Q15: What is a blue sky law?<br>A) It
Q17: _ is the highest duty of good
Q18: What is the balanced redox equation that
Q18: Which of the following correctly describes a
Q21: Under the Fair Credit Reporting Act, an
Q22: The due-on-sale clause prevents assumption of a
Q41: Which of the following is a Lewis