Given the equation A(g) 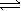 B(g) + 2C(g) . At a particular temperature, K =
B(g) + 2C(g) . At a particular temperature, K = 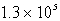 . If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?
. If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?
Definitions:
La Belle Indifference
A term referring to an unusual lack of concern for one’s physical symptoms, typically seen in conversion disorder.
Agoraphobia
An anxiety disorder involving intense fear and avoidance of places or situations where escape might be difficult or help might not be available in the event of panic.
Dissociation
A mental process that causes a lack of connection in a person's thoughts, memory, and sense of identity.
Physiological Basis
The underlying physical or biological mechanisms that contribute to a condition, behavior, or process.
Q11: Calculate the [H<sup>+</sup>] in a solution that
Q14: A particular radioactive element has a half-life
Q17: The number of unpaired electrons in an
Q18: The condensation product of two amino acids
Q21: What sugar is contained in RNA?
Q26: You have 3.00 L of a 3.48
Q26: Which of the following statements describes the
Q34: Write the equilibrium expression for the reaction<br>3O<sub>2</sub>(g)
Q42: SO<sub>2</sub><br>A) linear<br>B) trigonal planar<br>C) tetrahedral<br>D) pyramidal<br>E) V-shaped<br>
Q110: Which of the following has the smallest