Given the equation A(g) 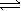 B(g) + 2C(g) . At a particular temperature, K =
B(g) + 2C(g) . At a particular temperature, K = 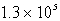 . If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?
. If you mixed 1.4 mol B, 0.050 mol C, and 0.0050 mol A in a 1-liter container, in which direction would the reaction initially proceed?
Definitions:
Pessimism
A tendency to focus on the negative aspects of life or to expect the worst possible outcome in any given situation.
Locus Of Control
A psychological concept referring to the extent to which individuals believe they have power over events in their lives.
Dr. David G. Myers
A psychologist known for his work in social psychology and happiness.
Positive Attitude
A mental state that involves looking at the good side of things and expecting positive results.
Q14: You have three sodium carbonate solutions on
Q21: N<sub>2</sub> is an example of a covalent
Q34: You have 3.00 L of a 3.00
Q44: Which of the following is not a
Q45: The Hall process for making aluminum involves<br>A)
Q47: When analyzing ideal gases, the temperature must
Q60: You have a sample of argon gas
Q65: Chlorine has how many electrons in its
Q89: If a lawsuit is filed in Quebec
Q117: A principle established in Donoghue v.Stevenson is