You have 3.00 L of a 3.00 M solution of NaCl(aq) called solution A. You also have 2.00 L of a 1.84 M solution of AgNO3(aq) called solution B. You mix these solutions together, making solution C. Calculate the concentration (in M) of 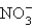 ions in solution C.
ions in solution C.
Definitions:
Conversion Value
The financial worth of a convertible security if it is converted into a different form, usually shares of the company's common stock.
Straight Bond Value
The value of a bond that pays fixed interest payments and does not have any additional features such as convertibility or callable options.
Conversion Value
The worth of a convertible security if it is converted into a different asset, usually common stock, at the current market price.
Convertible Bond
A type of bond that can be converted into a predetermined amount of the issuing company's equity at certain times during the bond's life, usually at the discretion of the bondholder.
Q1: In tort,damages as a remedy attempt to
Q14: Consider the following equilibrium: H<sub>2</sub>(g) + I<sub>2</sub>(s)
Q17: The number of unpaired electrons in an
Q41: For the reaction 2SO<sub>2</sub>(g) + O<sub>2</sub>(g) <img
Q44: Order the following bonds from the least
Q47: Discuss the role of insurance,especially compulsory insurance,and
Q68: Explain any limitation on the principle of
Q80: Rank the following bonds from least polar
Q128: In January,a driver accidentally caused a snowplow
Q131: Following a broadcast on the TV station