How many moles of O2(g) are needed to react completely with 51.2 L of CH4(g) at STP to produce CO2(g) and H2O(g) according to the following reaction? 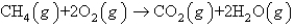
Definitions:
Global Trade Network
A complex and interconnected system of trade relationships, agreements, and channels that span across different countries and continents, facilitating the exchange of goods and services internationally.
Townshend Acts
A series of British laws passed in the late 1760s imposing duties on various goods imported to the American colonies, which sparked widespread protests and contributed to the American Revolutionary War.
Homespun Items
Goods made at home, typically referring to clothing or textiles that are handwoven or crafted, often symbolizing self-reliance or economic protest.
Successfully Resisted
The act of effectively opposing or standing firm against an opposing force or influence.
Q3: Which of the following is true when
Q4: Which of the following has the electron
Q6: In lecture, the professor named a molecule
Q15: State the maximum number of electrons allowed
Q19: What is the pH of a solution
Q29: Which statement is true of the
Q50: Perform the indicated conversion: 1.345 kcal =_
Q52: What mass of solute is contained in
Q68: The oxidation state of chlorine in ClO<sup>-</sup>
Q70: Calculate the molecular formula of a compound