How many moles of O2(g) are needed to react completely with 51.2 L of CH4(g) at STP to produce CO2(g) and H2O(g) according to the following reaction? 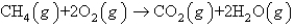
Definitions:
Annual Coupon
The yearly interest payment made to bondholders, based on the bond's face value.
Yield to Maturity
The total return expected on a bond if it is held until the date it matures.
Maturity Risk Premium
The additional yield that investors demand to compensate for the risk of holding a longer-term debt instrument, over and above the risk of short-term instruments.
Default Risk
The risk that a borrower will not make the required payments on their debt obligations, leading to a default.
Q4: The tertiary structure of a protein refers
Q11: Which of the following has the greatest
Q11: A balanced chemical equation is one that
Q16: Which of the following statements is not
Q25: Consider the following equilibrium: 2H<sub>2</sub>(g) + X<sub>2</sub>(g)
Q36: Draw the Lewis structures for the following
Q56: Perform the following conversion of pressure units:
Q77: How many resonance structures are there for
Q91: Calculate the mass of 3.663 moles of
Q106: Which one of the following species has