Consider the drawings below: 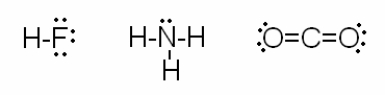 Which of the following statements are true? I. The electrons in each molecule tend to be attracted to the most electronegative element.
Which of the following statements are true? I. The electrons in each molecule tend to be attracted to the most electronegative element.
II) Each molecular drawing follows the localized electron model.
III) Both HF and CO2 are linear molecules and therefore nonpolar.
IV) The bond angles of NH3 are slightly less than 109.5o because the lone pair compresses the angles between the bonding pairs.
Definitions:
Uniform Residential
This refers to standardized forms or regulations pertaining to residential transactions or agreements, often aimed at simplifying legal processes.
Landlord and Tenant Act
Legislation that outlines the rights and obligations of landlords and tenants, aiming to ensure fair practices, safety, and habitability in rental agreements.
Attorney's Fees
The fees charged by lawyers for their legal services, which may be set as a fixed amount, an hourly rate, a percentage of any money awarded, or on a contingency basis.
Exclusive Possession
A legal right allowing an individual or entity to use a property without interference from the owner or any third parties.
Q8: Choose the correct electron configuration for nitrogen
Q10: Would NH<sub>3</sub> be classified as ionic or
Q18: The lowest energy level to allow f
Q26: Which species is the reducing agent?<br>A) Ti<br>B)
Q26: A minimum energy called the activation energy
Q29: Energy can be classified as either potential
Q30: Esters often have sweet, fruity odors.
Q37: The P<sub>vap</sub> for water at 100.0°C is<br>A)
Q43: In the presence of ultraviolet light, the
Q76: What element is reduced?