The specific heat capacity of aluminum is 0.89 J/g·°C. Calculate the amount of energy needed to warm 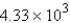 g of aluminum from 73.0°C to 155.0°C.
g of aluminum from 73.0°C to 155.0°C.
Definitions:
Phonemes
The smallest units of sound in a language that can distinguish one word from another.
Meaningful Units
Segments of information that have been organized in a way that gives them significance or purpose, such as syllables within words or phrases within sentences.
Phonemes
The smallest unit of sound in a language that can distinguish words.
Grice's Maxim
A set of guidelines proposed by Paul Grice that describe how effective communication is achieved through cooperation, including the maxims of quantity, quality, relation, and manner.
Q8: A 51.1-g sample of aluminum at 95.0°C
Q12: The name for <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6421/.jpg" alt="The name
Q23: A hydrogen balloon is at 25<sup>o</sup>C, 1.00
Q28: Which one of the following atoms has
Q36: Express <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6421/.jpg" alt="Express in
Q52: Write the electron configuration for Ca.
Q55: You are playing with a helium balloon
Q59: The name for NH<sub>4</sub><sup>+</sup> is _.
Q80: Determine the percentage composition (by mass) of
Q185: Titanium(IV) oxide has the formula<br>A) Ti<sub>4</sub>O<br>B) TiO<sub>4</sub><br>C)