Consider the reaction: 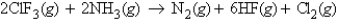 When calculating the
When calculating the 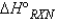 , why is the
, why is the 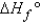 for
for 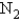 not important?
not important?
Definitions:
Franklin Roosevelt
The 32nd President of the United States (1933-1945), known for leading the country during the Great Depression and World War II through his New Deal policies.
Winston Churchill
A prominent British statesman who served as the Prime Minister of the United Kingdom during World War II and again in the early 1950s, known for his leadership and oratory skills.
Soviet Union
A socialist state in Eurasia that existed from 1922 to 1991, officially known as the Union of Soviet Socialist Republics (USSR).
Airlifting Supplies
The process of transporting goods or supplies by aircraft, typically used in emergency situations or to access remote areas.
Q6: A solution with a pH of 2
Q27: The name for Ag<sub>2</sub>CO<sub>3</sub> is _.
Q50: Two moles of gas A spontaneously convert
Q57: The electron configuration for Ca<sup>2+</sup> is identical
Q77: The name for (NH<sub>4</sub>)<sub>2</sub>CO<sub>3</sub> is _.
Q83: A 3.1-mol sample of KClO<sub>3</sub> was decomposed
Q83: The name for Zn(OH)<sub>2</sub> is _.
Q99: Order the elements Te, I, and Br
Q101: Would NaBr be classified as ionic or
Q122: The name for N<sub>2</sub>S<sub>3</sub> is _.