Consider the reaction: 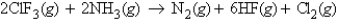 When calculating the
When calculating the 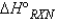 , why is the
, why is the 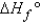 for
for 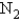 not important?
not important?
Definitions:
Passive
Accepting or allowing what happens or what others do, without active response or resistance.
Communication Style
The manner in which individuals express themselves and interact with others, which can vary greatly depending on personality, culture, and context.
Terminal Lung Cancer
An advanced stage of lung cancer where the disease is considered incurable and end-of-life care is the primary focus.
Myocardial Infarction
A medical condition commonly known as a heart attack, caused by the interruption of blood flow to part of the heart, resulting in heart muscle damage.
Q11: An aqueous solution of calcium nitrate is
Q16: Write the molecular equation, the complete ionic
Q34: A NaCl(aq) solution is automatically buffered.
Q39: Write the electron configuration for B.
Q42: Which has the higher [H<sup>+</sup>], 0.40 M
Q65: Chlorine has how many electrons in its
Q69: How many moles of Ca atoms are
Q73: In the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6421/.jpg" alt="In the
Q114: The correct formula for the carbonate ion
Q124: The compound PI<sub>3</sub> is named<br>A) potassium iodide<br>B)