The rusting of iron is represented by the equation 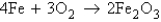 . If you have a 1.45-mol sample of iron, how many moles of Fe2O3 will there be after the iron has rusted completely?
. If you have a 1.45-mol sample of iron, how many moles of Fe2O3 will there be after the iron has rusted completely?
Definitions:
Current Ratio
A liquidity ratio that measures a company's ability to pay short-term obligations or those due within one year, calculated by dividing current assets by current liabilities.
Permanent Accounts
Balance sheet accounts that carry their ending balances over into the next accounting period, such as assets, liabilities, and equity accounts.
Accumulated Depreciation-Equipment
The total depreciation that has been recorded against a company's equipment since it was put into service.
Depreciation Expense-Equipment
The allocation of an equipment's cost over its expected useful life to reflect its decrease in value over time.
Q2: What is the normality of 1.00 L
Q22: When the following equation is balanced
Q30: Classify the following reaction: HNO<sub>3</sub>(aq) +
Q37: The specific heat capacity of silver is
Q40: Methane, CH<sub>4</sub>, the major component of natural
Q41: The frequency of the wave is the
Q55: _ is a phenomenon that may be
Q135: The name for the acid HNO<sub>2</sub> is<br>A)
Q180: The correct formula for lead(II) sulfite is<br>A)
Q200: The name for <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6421/.jpg" alt="The name