Multiple Choice
Which of the following reaction mixtures would produce the greatest amount of product, assuming all went to completion? Each involves the reaction symbolized by the equation 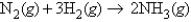
Definitions:
Related Questions
Q5: A 6.75-g sample of gold (specific heat
Q17: The normal freezing point of water is<br>A)
Q33: Draw the Lewis electron structure for the
Q34: A sample of helium gas occupies 14.3
Q39: Draw the Lewis structure for SiH<sub>4</sub>.
Q70: Calculate the molecular formula of a compound
Q82: Which of the following statements are true
Q89: A 7.33-g sample of lanthanum, La, combines
Q109: Complete the table by giving the predicted
Q208: Write the correct formula for acetic acid.