Calculate the mass of water produced when 9.47 g of methane, CH4, reacts with an excess of oxygen in the following unbalanced reaction. 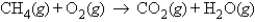
Definitions:
Total Liabilities
The aggregate of all debts and financial obligations a company owes to outside parties, reflected on the balance sheet.
Total Equity
The total value of an entity's assets after subtracting its liabilities, representing the owners' residual interest in the company.
Straight-Line Method
A method of calculating depreciation or amortization by evenly spreading the cost of an asset over its useful life.
Interest Semiannually
A payment structure for bonds or loans where interest payments are made twice a year.
Q1: Balance the equation<br>Na(s) + H<sub>2</sub>O(l) <span
Q2: What is the normality of 1.00 L
Q3: Which of the following statements is not
Q27: Draw the Lewis structure for KCN.
Q29: How many atoms of aluminum can be
Q42: How many molecules of F<sub>2</sub> are there
Q58: Balance the equation<br>Mg(OH)<sub>2</sub>(aq) + 2HBr(aq)
Q68: Balance the equation<br>NaBH<sub>4</sub> + BF<sub>3</sub>
Q83: The name for Zn(OH)<sub>2</sub> is _.
Q158: The name for CuCl<sub>2</sub> is _.