Calculate the mass of carbon dioxide produced from 22.3 g of octane, C8H18, in the following reaction. 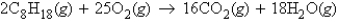
Definitions:
Productivity
The measure of the efficiency of production, often quantified as the ratio of output to inputs in the production process.
Incentives
Motivators or rewards that influence the actions or performance of individuals or organizations towards achieving certain goals or desired behaviors.
Alcohol Consumption
The intake of beverages containing ethanol, which can have social, cultural, or personal significance.
Marginal Cost
Marginal cost is the cost of producing one additional unit of a product or service, vital for pricing and production decisions.
Q6: Magnesium reacts with oxygen to form<br>A) MgO<br>B)
Q12: Vinegar contains carbon, hydrogen, and oxygen with
Q15: A solution has [H<sup>+</sup>] = 4.3 *10<sup>-</sup><sup>8</sup>
Q22: At 1 atm of pressure and a
Q23: Which of the following atoms has the
Q27: Draw the Lewis structure for KCN.
Q51: Write the electron configuration for Al.
Q59: Which of the following is the highest
Q67: The energy levels of the hydrogen atom
Q67: Is the molecule CF<sub>4</sub> nonpolar or polar?