Consider the following reaction at 1.10 atm and 19°C: 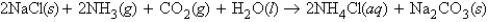 0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?
0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?
Definitions:
Income Received
It refers to the total amount of money obtained by individuals or entities typically through employment, investments, or other sources.
Upper Quintiles
Refers to the top 20% of a population in terms of income or wealth distribution.
Line L
Represents the relationship between the quantity of labor hired and the amount of output produced, often used in economics to illustrate productivity.
Income Received
The total amount of money received by an individual or entity during a specified time frame, from all sources.
Q3: Write the correct formula for calcium hydrogen
Q17: When the following equation is balanced,
Q32: How many moles of Al<sub>2</sub>O<sub>3</sub> are present
Q34: A compound is analyzed and found to
Q35: Of the following substances, choose the one
Q37: You transfer a sample of gas at
Q39: The empirical formula for a compound is
Q46: CH<sub>4</sub> has ionic bonds.
Q84: What is the molar mass of nitroglycerin,
Q214: Give the formula for silver(I) cyanide.