Consider the following unbalanced equation: 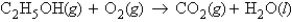 If 1.86 g of ethanol reacts with 14.3 g of oxygen, how many moles of water are produced?
If 1.86 g of ethanol reacts with 14.3 g of oxygen, how many moles of water are produced?
Definitions:
Excise Tax
A tax imposed on specific goods, services, or transactions, often with the aim of reducing their consumption or raising government revenue.
Lung Cancer
A type of cancer that originates in the lungs, often associated with smoking and exposure to hazardous pollutants.
Benefits-Received Tax
A taxation principle where taxes are levied on individuals based on the benefits they receive from the government's use of the collected tax.
Vertical Equity
A principle in taxation that proposes taxpayers with a greater ability to pay, such as higher income levels, should pay more in taxes than those with lesser ability to pay.
Q4: Sodium metal reacts with water to produce
Q11: A gas is collected over water at
Q21: N<sub>2</sub> is an example of a covalent
Q32: For the reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6421/.jpg" alt="For the
Q33: A d sublevel can hold a maximum
Q55: Consider the following standard heats of formation:
Q59: Would CO<sub>2</sub> be classified as ionic or
Q60: N<sub>2</sub>(g) + O<sub>2</sub>(g) <span class="ql-formula"
Q85: The yet undiscovered element with atomic number
Q192: Give the formula for potassium iodide.