Multiple Choice
For the reaction 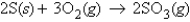 how many moles of SO3 can be produced from 7.2 g O2 and excess S?
how many moles of SO3 can be produced from 7.2 g O2 and excess S?
Definitions:
Related Questions
Q2: Which element or ion listed below has
Q4: The empirical formula for acetic acid is
Q8: The F<sup>-</sup> and O<sup>2-</sup> ions have the
Q10: The bonding forces that hold the atoms
Q14: You have three sodium carbonate solutions on
Q16: Determine the pressure exerted by 2.26 mol
Q53: Balance the equation<br>Fe(NO<sub>3</sub>)<sub>2</sub>(aq) + H<sub>2</sub>S(g)
Q106: Which of the following has the greatest
Q119: Calculate the mass of 23.7 moles of
Q141: The name for Cu<sub>3</sub>(PO<sub>4</sub>)<sub>2</sub> is _.