Fe2O3 (molar mass = 159.7 g/mol) reacts with CO (molar mass = 28.0 g/mol) according to the equation 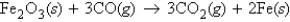 When 470.0 g Fe2O3 reacts with excess CO, what number of moles of Fe (iron) is produced?
When 470.0 g Fe2O3 reacts with excess CO, what number of moles of Fe (iron) is produced?
Definitions:
Corporation
A legal entity that is separate from its owners, with its own rights, privileges, and liabilities distinct from those of its members.
Director's Duties
Obligations that directors are expected to fulfill, including acting in the company's best interests and with due care.
Corporation
A legal entity recognized by law, separate from its owners, with the ability to own assets, incur liabilities, and conduct business.
Dividends
A portion of a company's earnings distributed to its shareholders, usually in the form of cash or additional stock.
Q20: The molar heat of fusion of water
Q21: A 4.06-L sample of carbon monoxide is
Q23: Which of the following atoms has the
Q30: In the equation <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB6421/.jpg" alt="In the
Q41: A compound contains 25.94% N and 74.06%
Q53: Fe<sub>2</sub>O<sub>3</sub> (molar mass = 159.7 g/mol) reacts
Q66: What mass of solute is contained in
Q76: Gaseous chlorine is held in two separate
Q76: Balance the equation<br>Pb(NO<sub>3</sub>)<sub>2</sub>(aq) + K<sub>2</sub>CrO<sub>4</sub>(aq)
Q79: Calculate the mass of 3.53 *10<sup>26</sup> atoms