How many moles of SbCl3 is formed when 4.00 mol Sb are reacted with 4.43 mol Cl2 according to the unbalanced equation 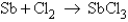
Definitions:
Q1: A mole ratio is used to convert
Q5: You have a partially filled party balloon
Q11: Which of the following has the highest
Q20: Which of the following is ranked in
Q29: An aqueous solution of lead(II) nitrate is
Q36: If you mix 40.0 mL of a
Q56: What element has the electron configuration 1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>4s<sup>2</sup>3d<sup>10</sup>4p<sup>6</sup>5s<sup>2</sup>4d<sup>10</sup>5p<sup>6</sup>6s<sup>2</sup>4f<sup>14</sup>5d<sup>10</sup>6p<sup>5</sup>
Q77: The molar mass of barium nitrate, Ba(NO<sub>3</sub>)<sub>2</sub>
Q135: The name for the acid HNO<sub>2</sub> is<br>A)
Q183: Give the formula for mercury(II) chloride.