Look at the reaction below: 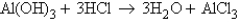 Suppose 0.55 g of water were produced from 1.2 g of aluminum hydroxide and a certain amount of hydrochloric acid. Which of the following statements is true? Choose the best answer.
Suppose 0.55 g of water were produced from 1.2 g of aluminum hydroxide and a certain amount of hydrochloric acid. Which of the following statements is true? Choose the best answer.
Definitions:
Breathing
The physiological process of inhaling oxygen from the air into the lungs and exhaling carbon dioxide out, essential for life.
Ventilation-Perfusion Coupling
The coordination between the air flow in the lungs (ventilation) and the blood flow in the circulatory system (perfusion), essential for efficient gas exchange.
Alveoli
Tiny air sacs within the lungs where the exchange of oxygen and carbon dioxide occurs, crucial for breathing and systemic respiration.
Blood Flow
The continuous circulation of blood through the cardiovascular system, delivering oxygen and nutrients to and removing waste products from tissues.
Q3: The largest a party balloon can get
Q6: Pb(s) + AgNO<sub>3</sub>(aq) <span class="ql-formula"
Q38: How many d electrons are in an
Q41: A compound contains 25.94% N and 74.06%
Q47: The empirical formula of a compound is
Q50: The name for the compound Fe<sub>2</sub>O<sub>3</sub> is
Q51: You are holding two balloons of the
Q53: An aqueous solution of potassium chloride is
Q70: For the reaction of C<sub>2</sub>H<sub>4</sub>(g) with O<sub>2</sub>(g)
Q108: Write the correct formula for beryllium hydride.