Ammonia reacts with oxygen to form nitrogen dioxide and water according to the following equation: 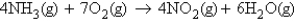 You react ammonia and oxygen, and at the end of the experiment you find that you produced 23.0 g of water and have 8.52 g of ammonia left over. Determine the mass of oxygen reacted.
You react ammonia and oxygen, and at the end of the experiment you find that you produced 23.0 g of water and have 8.52 g of ammonia left over. Determine the mass of oxygen reacted.
Definitions:
Intelligence Tests
Tests created to evaluate human cognitive skills and intelligence levels in a uniform manner.
Disorganized Attachment
A type of insecure attachment seen in some children who display confused or contradictory behaviors towards caregivers, often resulting from trauma or neglect.
Odd Behaviors
Actions or reactions that deviate from what is considered normal or expected in a specific cultural or social context.
Pinching
The act of squeezing the skin and flesh between the thumb and a finger, which can be gentle or forceful.
Q19: Write and balance the equation showing the
Q31: The formula of the compound formed in
Q42: The name for the acid H<sub>2</sub>SO<sub>3</sub> is<br>A)
Q44: The name for the acid HNO<sub>3</sub> is
Q55: Consider the following standard heats of formation:
Q66: Balance the equation<br>Sb(s) + O<sub>2</sub>(g)
Q73: The correct formula for iron(II) phosphide is<br>A)
Q89: A 7.33-g sample of lanthanum, La, combines
Q94: What is the molar mass of Li<sub>2</sub>SO<sub>4</sub>?<br>A)
Q161: The name for HCN(g) is _.