Consider the following reaction at 1.10 atm and 19°C: 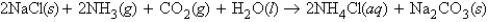 0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?
0.228 mol of sodium chloride, 3.03 L of ammonia, 2.00 L of carbon dioxide, and an unlimited amount of water react to form aqueous ammonium chloride and solid sodium bicarbonate. How many moles of ammonium chloride are formed in the reaction?
Definitions:
Social Exclusion
A process where individuals or groups are systematically blocked from rights, opportunities, and resources that are normally available to members of society and necessary for social integration.
Coveted Internship
A highly sought-after temporary position, often in a prestigious organization or field, that offers valuable experience and networking opportunities.
Public Office
A position of authority or service in the government, elected or appointed, which involves fulfilling certain duties to the public.
Manifest Function
The recognized and intended consequences of any social pattern, contributing to societal equilibrium.
Q3: You have a 16.0-oz. (473-mL) glass of
Q15: Covalent bonding occurs when electrons are shared
Q23: Determine the enthalpy change when 19.4 g
Q44: The name for the acid HNO<sub>3</sub> is
Q45: An aqueous solution of ammonium carbonate is
Q50: Which of the following statements about the
Q64: The most electronegative element of those listed
Q65: Consider a reaction given by the
Q102: The correct formula for ammonium dichromate is<br>A)
Q166: The correct formula for sodium sulfide is<br>A)